Parler
Parler Gab
Gab
Why are direct-to-consumer drug ads allowed at all?
In its ruling, the FDA stipulated that Big Pharma must now be "clear, conspicuous and neutral" in all drug advertising in lieu of presenting emotional appeals as they currently do, misleading customers about the true attributes of the drug or drug-related service being offered. "Information must be clear and in a language that is readily understandable by consumers," the new FDA rules state. "Audio must be understandable in terms of volume, articulation and pacing. Television ads must include text and audio, and the text displayed must match the audio, be shown for long enough and be easy to read." "Advertising must not include elements, such as statements, text, images or sounds, that distract from the main message of the ad. Any text used must be large enough and in an easily-readable font. Notably, ads no longer need to explain how a drug works or what its benefits are. Simply listing major side effects and conditions preventing use is sufficient, according to the ruling." The ruling is actually an update from an early decision in 2010 stipulating that drug ads need to be presented to consumers in easily understandable language using text that is easy to read – many drug commercials still put the side effects in very small font, very quickly, at the end of the commercial where most people miss it. It is said that Americans see up to 10,000 ads daily, a truly astounding number. And with something as serious as pharmaceutical drugs available by prescription only being included among them, it is important that consumers get only the most accurate information that does not mislead or trick them. Big Pharma is notorious for lying to consumers about the safety, or lack thereof, of pharmaceuticals. The FDA would have done well to deal with this problem decades ago, but perhaps it is better late than never? In 2021, the pharmaceutical industry spent $6.88 billion on direct-to-consumer pharmaceutical ads – ads that are not allowed in many other countries at all, by the way. This sounds like a lot of money, but it is a drop in the bucket for the multi-trillion-dollar pharmaceutical industry, which is arguably the world's biggest racket. "There's a lot at stake when consumers don't understand contraindications," one report about the matter explains. "Making those clear protects people accessing telehealth services, which increasingly prescribe remotely." "Consumers should always consult doctors and pharmacists first to understand interactions and risks when taking new medications." In the comments, others added to this that drug companies have no business advertising their drugs directly to consumers period, suggesting that the practice should be outlawed entirely. More related news about the FDA can be found at FDA.news. Sources for this article include: TheEpochTimes.com NaturalNews.comTexas judge probed for funneling $11M in taxpayer money to ally who has ties with prominent Dems
By Belle Carter // Share
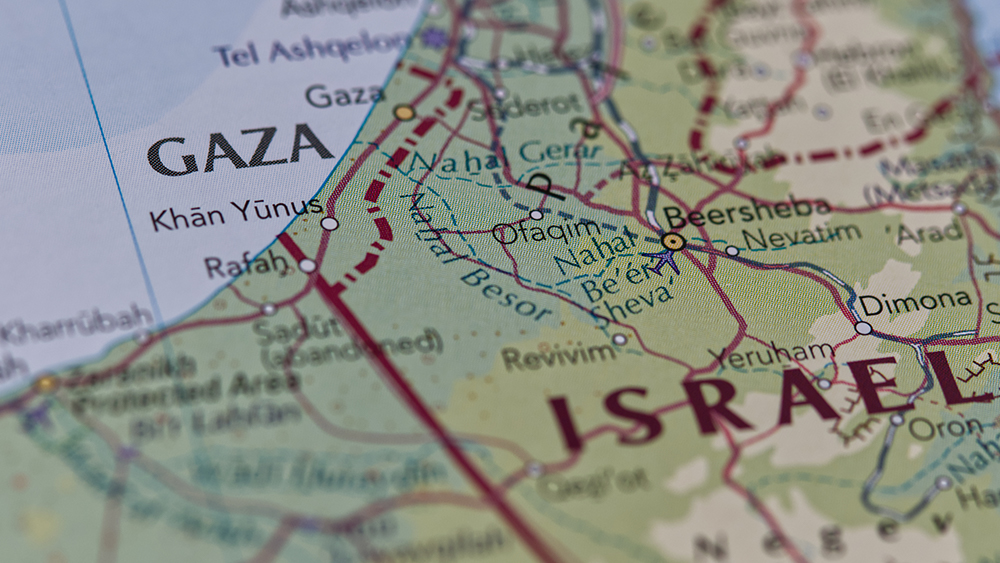
Netanyahu’s CORRUPTION trial resumes amid conflict in the Gaza Strip
By Ramon Tomey // Share
Peter Koenig: Israel wants CONTROL of water, hydrocarbons in the Middle East
By Kevin Hughes // Share
Biometric data and surveillance: DNA being eyed as the “ultimate global ID”
By Zoey Sky // Share
Judge rejects special counsel’s request to seal documents in Trump’s classified materials case
By News Editors // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share